Article
Novavax’s Long-Awaited COVID-19 Vaccine Authorizations Offer an Alternative to mRNA
European Commission and World Health Organization approvals usher in key addition to vaccine supply
Science,
2021
Read or listen offline
1×
Recommendation
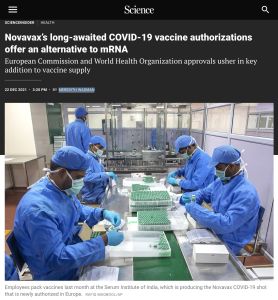
Expanding distribution of a new vaccine produced by Maryland biotech firm Novavax, recently authorized for emergency use, means jab-hungry countries may finally start gaining control of COVID-19. Besides offering efficacy roughly equivalent to that of mRNA vaccines, Novavax’s more traditional “dead-virus” version doesn’t require super cold storage, making it more suitable for use in the world’s poor, remote regions.
Take-Aways
About the Author
Meredith Wadman, MD, joined Science as a staff writer in September 2016. She has been a staff writer for Nature and a contributing writer at Fortune. Her first book was The Vaccine Race: Science, Politics and the Human Costs of Defeating Disease.

















Comment on this summary or Начать обсуждение